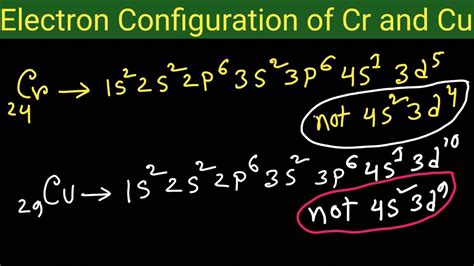
cr electron configuration|electron configuration for dummies : Tuguegarao The chemical symbol for Chromium is Cr. Electron Configuration and Oxidation States of Chromium. Electron configuration of Chromium is [Ar] 3d5 4s1. .
DLTB Pasay Tramo Address: M. Dela Cruz, Malibay, Pasay, Metro Manila [Get Direction] DLTB Terminal Cubao Address: 821 Aurora Blvd, Cubao, Quezon City, Metro Manila [Get Direction] DLTB Terminal Turbina Address: DLTB, Turbina, Calamba, Laguna [Get Direction]
PH0 · ground state electron configuration chart
PH1 · full electron configuration of chromium
PH2 · electron configuration order of filling
PH3 · electron configuration for dummies
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · chromium ground state electron configuration
PH7 · choose the correct orbital diagram for chromium
PH8 · Iba pa
Aprenda a tocar a cifra de Esperando Na Janela (Cogumelo Plutão) no Cifra Club Você é a escada da minha subida / Você é o amor da minha vida / É o meu abrir de olhos do amanhecer / Você é a espera na janela / A ave que vem de longe tão bela / A esperança que arde em calor / Você é a tradução do que é o amor‘F4F’ is the short form for ‘follow for follow.’ It is an old Instagram growth strategy that people often use. It is an old Instagram growth strategy that people often use. Before Instagram regulated this practice, you could grow your account to thousands of followers in a short time without much effort.
cr electron configuration*******How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are .
To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of .
Subscribed. 1.4K. 121K views 4 years ago. The answer is [Ar] 4s1 3d5 Because half-filled subshells are somewhere stable, the atom prefers to have a half-filled . For example, the electron configurations of the transition metals chromium (Cr) and copper (Cu), are not those we would expect. Rather, Cr and Cu take on half . Learn the correct electron configuration of chromium ([Ar]3d54s1) and why it differs from the ideal order. See answers and explanations from chemistry experts and users on Socratic. The chemical symbol for Chromium is Cr. Electron Configuration and Oxidation States of Chromium. Electron configuration of Chromium is [Ar] 3d5 4s1. . What Is The Electron Configuration of Chromium? So, with the same process, the electron configuration of the Chromium will actually be . Cr's electron configuration, following the model would be: \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^23d^4\0, but instead it is \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^13d^5\), because .Learn how to write electron configurations for atoms using the Aufbau principle, Pauli exclusion principle, and Hund's rule. This web page is part of a free textbook on .The chemical symbol of Chromium is ‘Cr’ Electronic configuration of d-block. In general, the outer electronic configuration of these elements is (n-1) d 1-10 n s 1-2. Here, (n–1) stands for the inner d orbitals which may have one to ten electrons, and the outermost ns orbital may have one or two electrons. Electronic configuration of Cr Chromium is a chemical element with atomic number 24 which means there are 24 protons and 24 electrons in the atomic structure.The chemical symbol for Chromium is Cr. Electron Configuration and Oxidation States of Chromium. Electron configuration of Chromium is [Ar] 3d5 4s1. Possible oxidation states are +2,3,6. . The electron configuration for chromium is NOT #1s^2 2s^2 2p^6 3s^2 3p^6 3d^4 4s^2#, but #color(blue)(1s^2 2s^2 2p^6 3s^2 3p^6 3d^5 4s^1)#.. Interestingly enough, Tungsten is more stable with an . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .

An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .electron configuration for dummies An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation .Chromium is a chemical element with atomic number 24 and represented by the symbol Cr in the Periodic Table. Chromium is a lustrous, hard metal that has a silver-grey colour. It has a high melting point. The electron configuration of chromium is [Ar]3d 5 4s 1, which can be explained by the stability offered by a half-filled d-orbital.
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
Cr and Cu are the two exceptions of electron configuration of atoms up to Kr. this is because a 1/2 or completely full D block has extra stability, therefore in the case of Chromium .The electronic configuration of chromium is 1s2 2s2 2p6 3s2 3p6 4s1 3d5. The first five subshells contain the core electrons of chromium and can be condensed to the symbol for argon. The valence electrons of chromium are found in the 4s and 3d .Chromium electron configuration. Cr (Chromium) is an element with position number 24 in the periodic table. Located in the IV period. Melting point: 1857 ℃. Density: 7.14 g/cm 3 . The order of filling the orbitals with electrons in the Cr atom is an exception to the rule. Expected electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d4 But in .
Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 52 Cr Electron configuration [Ar] 3d 5 4s 1 CAS number: 7440-47-3 ChemSpider ID:cr electron configuration Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can .In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.cr electron configuration electron configuration for dummiesIn this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
Electron Configuration of Chromium. Mr. Causey shows you step by step how to write the electron configuration and orbital notation for chromium (Cr). Remembe.
There are several important exceptions to the general pattern for electron configurations of the elements. In this video we will look four different exceptio.
Watch Bitchinbubba Leaked Onlyfans Porn Videos Free 4k Sex - Big Tits, bitchinbubba, rocky, bitchinbubba rocky, Blowjob, #rocky, #bitchinbubba, public, #bj, beach .
cr electron configuration|electron configuration for dummies